Trending...
- San Antonio Board of REALTORS® Among First to Use Passkey Login with Solid Earth's SSO Dashboard - 136
- Alpha Modus Files 7th IP Action Against Rackspace Following $3M CEO Investment and Strategic Partnership Expansion
- Colorado Springs Police Department's K9s Blue, Loki, and Nyx have Received Donations of Body Armor
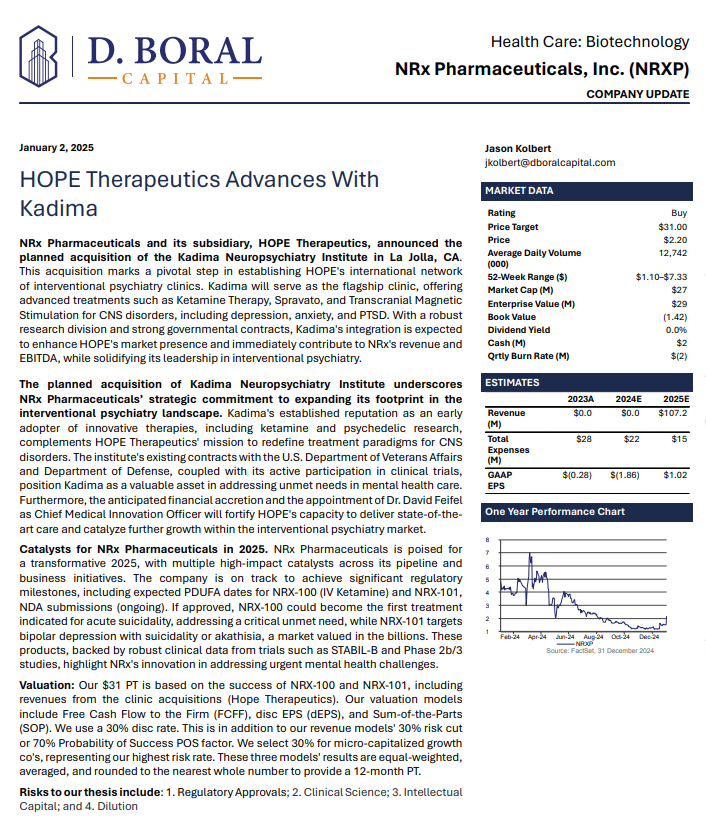
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) $NRXP Has $7.8 Million in Financing for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - ColoradoDesk -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
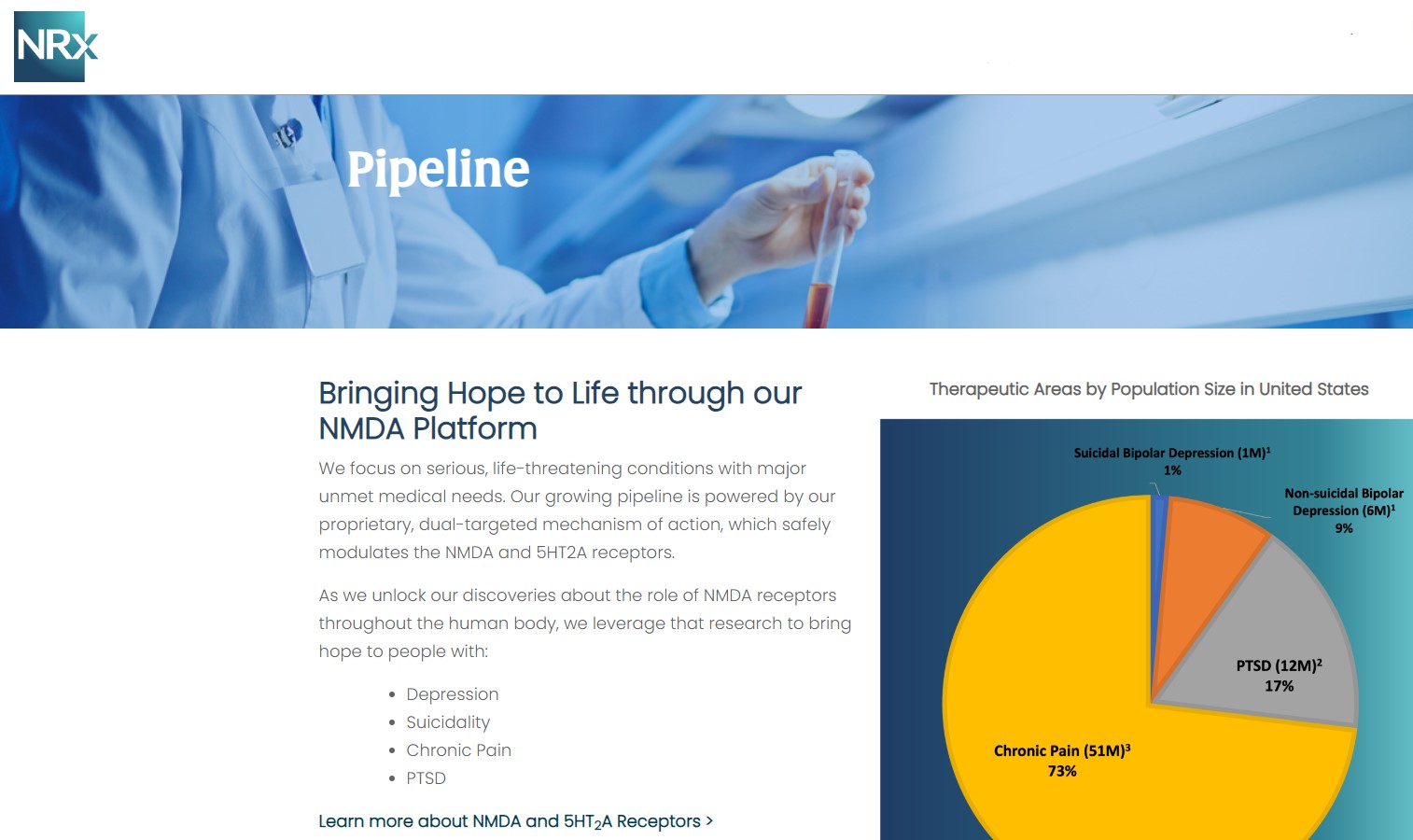
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
More on Colorado Desk
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Abbreviated New Drug Application (ANDA) for Preservative-Free IV Ketamine
On June 5th NRXP announced the transmission of its Abbreviated New Drug Application (ANDA) for electronic filing to the U.S. Food and Drug Administration (FDA) for NRX-100, its preservative-free IV ketamine formulation, for use in all existing approved indications such as anesthesia and pain management.
NRXP anticipates filing a citizen's petition with the FDA to remove benzethonium chloride, a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. NRXP believes that the preservative-free feature of NRX-100 will be deemed of benefit to patients because of the known toxicity of closely related benzalkonium chloride in current drug products. Preservatives were originally added to sterile injectable products in an era when a single vial of medication was used to treat multiple patients, a practice no longer allowed in US hospitals. NRXP has demonstrated that there is no need for such preservatives to maintain stability and sterility in ketamine presentations intended for single-patient use. Should the citizen's petition be granted, all formulations of ketamine sold in the US could face a regulatory requirement to be preservative free.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
More on Colorado Desk
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
More on Colorado Desk
- Michael Reafsnyder opens solo exhibition at Scott Richards Contemporary Art in San Francisco
- Colorado: Gov. Polis Urges House to End the Madness and Do Right by Coloradans by Starting Over on Federal Bill
- Valley Sleep Therapy Expands to Prescott with New Location at Crossings Road
- $17.4 Million Total Revenue for First Half of 2025 (up 31.8% YOY) for Global Wet Trades Services Provider with High Value Bitcoin Investments
- $12.8 Million Net Revenue for 2024 for Cloud-Based Crowdsourcing Recruitment and SaaS-Enabled HR Solutions Provider: Baiya International Group Inc
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Abbreviated New Drug Application (ANDA) for Preservative-Free IV Ketamine
On June 5th NRXP announced the transmission of its Abbreviated New Drug Application (ANDA) for electronic filing to the U.S. Food and Drug Administration (FDA) for NRX-100, its preservative-free IV ketamine formulation, for use in all existing approved indications such as anesthesia and pain management.
NRXP anticipates filing a citizen's petition with the FDA to remove benzethonium chloride, a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. NRXP believes that the preservative-free feature of NRX-100 will be deemed of benefit to patients because of the known toxicity of closely related benzalkonium chloride in current drug products. Preservatives were originally added to sterile injectable products in an era when a single vial of medication was used to treat multiple patients, a practice no longer allowed in US hospitals. NRXP has demonstrated that there is no need for such preservatives to maintain stability and sterility in ketamine presentations intended for single-patient use. Should the citizen's petition be granted, all formulations of ketamine sold in the US could face a regulatory requirement to be preservative free.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
More on Colorado Desk
- New Housing and Public Safety Laws Take Effect to Save Coloradans Money on Housing and Make Colorado Safer
- Morris Animal Foundation Names Ike Nicoll as Chief Executive Officer
- 20/20 Institute Announces Expansion with New State-of-the-Art LASIK Center in Colorado Springs
- Hire Virtue Announces Executive Sponsorship Opportunity for Houston Hiring Blitz & Job Fair on August 6, 2025
- Inked & Maxim Model Teisha Mechetti Turns Heads—And Builds Community Impact
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on Colorado Desk
- Spartan & Guardians Partner with Guitar Legend Buckethead to Support Global Child Rescue Efforts
- Preliminary.online Introduces Short-Term Job-Readiness Courses with Employer-Verified Certifications
- Psychologist-Turned-Hermeticist Releases Modern Guide to the Seven Hermetic Principles
- Winners Announced for Asia Pacific Business Awards 2024-2025
- Hamvay-Lang and Lampone.hu Join Forces with AIMarketingugynokseg.hu to Elevate Hungarian Lifestyle Brands on the Global Stage
- Google AI Quietly Corrects the Record on Republic of Aquitaine's Legal Sovereignty
- NYC Leadership Strategist Stacie Selise Launches Groundbreaking 4S Framework Series to Redefine Executive Excellence
- Colorado: Governor Polis Appoints Rory N. Devlin to the Elbert County Court in the 23rd Judicial District
- Governor Polis, Office of Economic Development and International Trade Celebrate Sale of EVRAZ North America including Rocky Mountain Steel in Pueblo, Welcomes Atlas Holdings to Colorado
- Colorado: Delivering Passenger Rail: Governor Polis Celebrates Major Step Towards Delivering Northern Front Range Passenger Rail
- Make Innovation Matter: Support H.R.1's R&D Expensing Relief for American Small Businesses
- Colorado Springs: Mayor certifies 2025 Special Election results
- Technologist Jay Harris Delivers Keynote for DenverDevDay
- Agreement to Supply US-Based Defense Provider with Thin-Film Solar Tech for Orbital Application; Ascent Solar Technologies, Inc. (N A S D A Q: ASTI)
- Jones Law Firm, PC Refreshes Denver Child Support Services Page to Better Serve Colorado Families
- Colorado: Governor Polis: U.S. Supreme Court Makes it More Difficult to Protect Constitutional Rights, Creates Chaos
- Colorado: Governor Polis, Hospital Leaders, Health Care Advocates: Devastating Cuts Should Be Removed From Federal Budget Bill
- Byrd Davis Alden & Henrichson Launches Independence Day Safe Ride Initiative with 500 Free Uber Credits
- Stonebridge and Stout NYC Hospitality Group Introduce New Era for The MC Hotel, Autograph Collection in Montclair, NJ
- databahn Launches GenAI Sales Intelligence Platform to Revolutionize Fortune 500 and Global 2000 Account Research