Trending...
- Governor Polis, Colorado Springs Leaders Urge Congress to Extend Health Care Tax Credits and Protect Families from Skyrocketing Costs
- Colorado: Governor Polis Highlights Looming Expiration of Federal Health Care Tax Credits
- MoArk Dental & Implants Introduces Yomi Robotic Technology for Implant Surgery
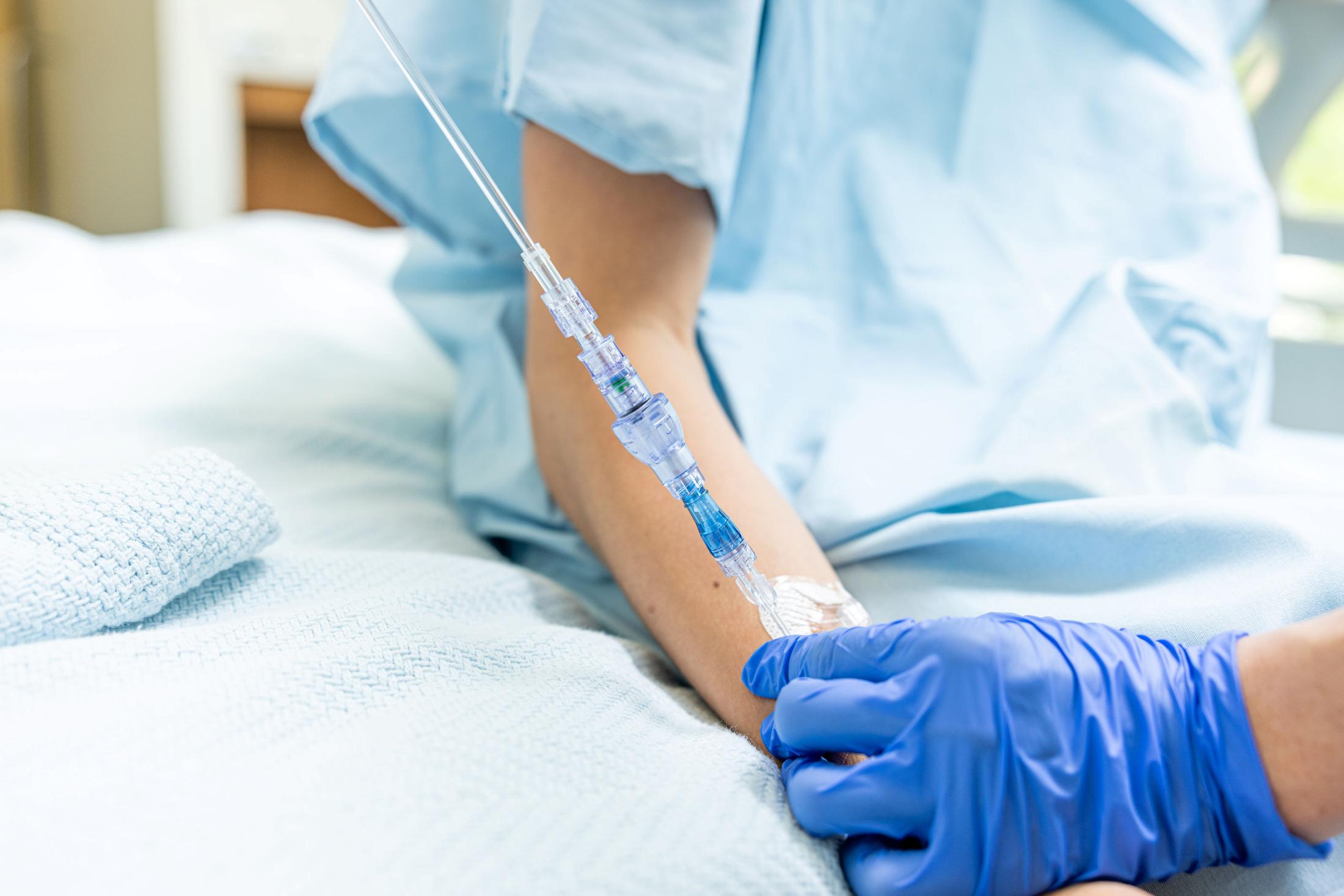
FAYETTEVILLE, Ark. - ColoradoDesk -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on Colorado Desk
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on Colorado Desk
- Colorado Springs: Join CSPD and Scheels for the Fifth Annual Balltoberfest!
- Colorado Springs: Last chance to experience Motorless Morning this year
- Celebrate Arts Month with the Colorado Springs Pioneers Museum
- Green Office Partner Secures the #1 Spot on Chicago Sun-Times' Best Workplace List Two Years Running
- AdvisorVault Releases New Explainer Video on their 17a-4 Managed 365 Service
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
Filed Under: Health, Technology
0 Comments
Latest on Colorado Desk
- Colorado: Polis Administration Announces Increased Incentives for Electric Vehicles
- SPOZZ, the Community-Owned Direct-to-Fan Music Ecosystem, adds "BEATS" — a Creator-to-Creator Marketplace
- DAECO Painting Sets the Gold Standard for High-End Interior Painting Services in Denver, CO
- Boston Industrial Solutions Expands Availability of Industry-Leading Primers to Mexico
- Unprescribed™ Introduces the Focus, Mood & Mind System™
- Colorado Springs: CSPD detectives make arrests in several connected robbery investigations from March 2025
- Mend Colorado Expands Spine Care in Lafayette to Help Residents Overcome Back and Neck Pain
- Texas Mechanic Unveils "Mighty Mule" Experimental Pontiac Engine—Delivering Over Triple the Factory Horsepower
- Colorado: Governor Polis & JBC Protect Food Support for Women and Children Ahead of Federal Shutdown, But Uncertainty Looms Despite State Action
- Colorado: Governor Polis Mourns the Passing of Conservation Icon Jane Goodall
- $20 Target in Noble Capital Markets Report Supported by Live Stream of 1ST Global Super League Kerala Event from AI Powered Sports Leader: $SEGG
- Vesica Health Granted PLA Billing Code for AssureMDx
- Colorado is Ranked 2nd Healthiest State in the Country
- Colorado: Governor Polis Gets Protected
- Colorado Springs: Notice of New Technology: CSPD speed safety cameras
- Colorado Springs: Second public meeting to be held for historic neighborhood park master plan
- Colorado: Governor Polis Warns Of Raising Costs on Americans Ahead of Federal Shutdown
- Newest Mako Smartrobotics™ System Used for First Time in the State Of Illinois for Total Joint Replacement Surgery
- $20 Million Annualized Revenue Projected from 20+ Acquisitions and Scaling of Top Quality Dental Labs Across Florida: Standard Dental Labs $TUTH
- Grok Wrote a Direct Message to Elon Musk Discussing Netverse & Phinge CEOs Challenge to Live Debate & Added "it'd be epic to see you two hash it out"